Abstract
This systematic review and meta-analysis compares sodium hyaluronate (HY) with non-HY based artificial tears in the treatment of dry eye syndrome. A literature search for clinical trials comparing HY against non-HY preparations was conducted across PubMed, Cochrane Central Register of Controlled Trials and Scopus databases from inception up to May 2016. Majority of the 18 studies selected for review showed superiority of HY in improving ocular staining and symptoms. Randomized controlled trials (RCTs) examining Schirmer’s I (SH) and tear breakup time (TBUT) underwent further meta-analyses with calculation of pooled standardized mean differences (SMDs) with 95% confidence intervals (CIs). 7 RCTs including 383 eyes randomized to HY and 596 eyes to non-HY preparations underwent meta-analysis for SH. 9 RCTs including 458 eyes randomized to HY and 651 eyes to non-HY preparations underwent meta-analysis for TBUT. By fixed-effects modelling, HY demonstrated greater improvement of SH compared to non-HY preparations (SMD, 0.238; 95% CI, 0.107 to 0.369; p < 0.001). By random-effects modelling, HY demonstrated less improvement of TBUT (SMD, −0.566; 95% CI, −1.099 to −0.0336; p = 0.037). In summary, neither preparation was shown to be consistently superior across all outcome measures. The difference in effect between preparations on SH and TBUT was not clinically significant.
Similar content being viewed by others
Introduction
The International Dry Eye Workshop1 defines dry eye syndrome (DES) as a multifactorial heterogeneous disease of the tear film and ocular surface that results in discomfort, visual acuity disturbance and tear film instability. It arises when there is disruption to the lid surface and function, cornea sensation, blink reflex, tear film production and stability, tear composition and drainage2, 3 leading to complications such as persistent corneal epitheliopathy and corneal infection. DES affects nearly 3.23 million women, 1.68 million men and 4.91 million Americans 50 years and older4.
A wide range of treatment options is available in the management of DES, including topical lubricants or artificial tear substitutes, ointments or gels, topical secretagogues, anti-inflammatory therapy, biologic tear substitutes, punctal occlusion, moisture chamber goggles and surgery. Artificial tears arguably provide the most affordable, immediate and least invasive form of relief. Previous generations include integrated natural polymers (such as cellulose derivatives) and synthetic polymers [(such as polyvinyl alcohol (PVA) and hydroxypropylguar (HP Guar)].
In recent decades, sodium hyaluronate has emerged as an option in artificial tear therapy. It owes its efficacy to hyaluronic acid (HY), a naturally occurring linear biopolymer consisting of repeating disaccharide units of N-acetyl-D-glucosamine and sodium-D-glucoronate. HY is widely used today and has been shown to result in both subjective and objective improvement of DES. A recent meta-analysis by Kong X. et al.5 compared the effect of HY and non-HY preparations in symptomatic relief of subjects and could not demonstrate superiority of one preparation over the other. However, the comparative efficacy of both artificial tears with regard to all other common outcome measures of DES remains unclear.
The aim of this systematic review and meta-analyses is to compare the efficacy of sodium hyaluronate against non-HY based preparations in the treatment of DES.
Results
Literature Retrieval Results
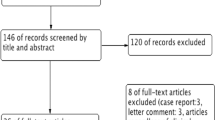
The initial literature search generated a total of 408 articles. 141 duplicates were removed. Based on the inclusion criteria, 247 articles were deemed unsuitable. Table 1 details the reasons for exclusion of studies. Of the remaining 20 studies, the full-text articles of 18 studies6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23 were successfully retrieved and included in the systematic review. Of these, 10 were RCTs which reported SH I and/or TBUT results6, 7, 9,10,11, 14, 18, 19, 21, 22 and underwent further meta-analyses. Figure 1 summarizes the study selection process.
Characteristics of Studies
A total of 18 studies, published between 1987 to 2012, were included in this review. Only 1 study was conducted in Asia, while the rest were conducted in Europe and the United States of America. The majority were parallel studies (12 studies) and the others were crossover studies (6 studies). All the parallel studies and 4 of the 6 crossover studies were randomized (crossover studies were randomized to which eyedrop subjects started with). 16 studies were single or double-masked, while the remaining 2 did not indicate if masking was carried out. The studies recruited subjects with DES of varying severities and followed them up to between 14 to 90 days. The preparations of HY ranged from 0.1% to 0.4% and most of the control eyedrops were MC-based (7 studies) or PVA-based (5 studies). Key study characteristics are summarized in Table 2.
Outcome measures examined across all studies are summarized in Table 3. TBUT, SH I, ocular staining and symptoms were the 4 most commonly studied outcome measures.
Risk of Bias Assessment
The risk of bias assessment of the 10 RCTs which underwent further meta-analyses are presented in Table 4. For selection bias, the risk for all the studies, with the exception of Condon et al.19 could not be determined due to the lack of information regarding the randomization sequence process and allocation concealment. 7 of the 10 studies were observer/investigator-masked and assessed to be at low-risk of detection bias. However, only 3 studies blinded study participants. Majority of studies (8 of 10) did not have any missing outcome data, or had missing data which was deemed insignificant for the study to be considered at high-risk of attrition bias. Although we did not have access to detailed study protocols, we assessed all the included studies to be at low-risk of selective reporting bias, given that all the outcomes described in their respective methodology sections were reported in their results.
Quantitative Analyses
SH I
7 RCTs6, 7, 10, 18, 19, 21, 22 were included in the meta-analysis for SH I outcomes (Fig. 2 and Table 5). A pooled total of 383 eyes were randomized to HY preparations and 596 eyes to non-HY preparations. The I2 value of 44.25% reflected acceptable heterogeneity, with corresponding symmetry of the funnel plot. By fixed-effects modelling, eyes in the HY group demonstrated greater improvement of SH compared to non-HY preparations (SMD, 0.238; 95% CI, 0.107 to 0.369), with results reaching statistical significance (p < 0.001).
TBUT
9 RCTs6,7,8, 10, 11, 14, 18, 21, 22 were were included in the meta-analysis for TBUT outcomes (Fig. 3 and Table 6). A pooled total of 446 eyes were randomized to HY preparations and 651 eyes to non-HY preparations. TheI2 value of 92.49% reflected significant heterogeneity, with corresponding skewing of the funnel plot. By random-effects modelling, eyes in the HY group demonstrated less improvement of TBUT compared to eyes in the non-HY group (SMD, −0.566; 95% CI, −1.099 to −0.0336), with results reaching statistical significance (p = 0.037).
From the funnel plot, the RCTs by Sanchez et al.11 and Benelli et al.10 were identified as outliers. However, upon re-examination of their methodologies, the authors found no justification for exclusion of these studies from the analysis. Nonetheless, a second meta-analysis excluding these 2 studies was run (Fig. 4 and Table 7). While there was greater symmetry of the funnel plot and a lowering of the I2 value, the I2 value remained above 50%. By random-effects modelling, eyes in the HY group now appeared to demonstrate more improvement of TBUT compared to eyes in the non-HY group (SMD, 0.00761; 95% CI, −0.232 to 0.247). However, the results were not statistically significant (p = 0.95).
Qualitative Analyses
Supplementary Tables S1–11 present the extracted data for all outcome measures examined in this analysis. Only outcome measures examined in 2 or more studies are described. Other outcomes examined by only one study and therefore not described in this paper include the tear evaporation rate8, tear turnover rate8, tear film stability8, tear prism height14, corneal autofluorescence16, tear clearance rate17, tear function index17, tear ferning pattern test18 and corneal sensitivity20.
Symptoms
15 studies6,7,8,9, 12,13,14,15,16, 18,19,20,21,22,23 reported symptoms as an outcome measure, with a total of 20 head-to-head comparison data sets. Majority of the control eyedrops were cellulose-based.
HY demonstrated superiority over control eyedrops in the majority of datasets (10 datasets), with 6 of the 10 demonstrating statistical significance. None of the 4 data sets which demonstrated superiority of the control eyedrop had statistical significance.
Symptoms in DES range from foreign-body sensation, grittiness, red eyes, mucoid discharge, irritation, sensation of dryness and tearing. Symptoms may be multifactorial and influenced directly by the volume (rather than the active component) of eyedrops. Symptoms such as ‘blurring of vision’ may be due also to the viscosity of eyedrops – cellulose and carbomer preprarations may be more viscous than others and cause transient blurring immediately post-instillation. The subjectivity of this outcome measure, wide-range of assessment methods, and inherent inter- and intra-subject variability pose challenges to any systematic review or meta-analysis. This has been attempted previously by Kong X. Y. et al.24, who were unable to demonstrate unequivocal superiority of HY over other eyedrops in providing subjective relief to DES patients.
Ocular Surface Staining
17 studies6,7,8,9,10,11,12,13,14,15, 17,18,19,20,21,22,23 examined ocular staining, with a total of 22 head-to-head comparison datasets. Studies included a range of stains and scoring systems, with examination of either the cornea, conjunctiva or both. If more than one stain was used, fluorescein staining results were extracted. If more than one area was examined, results from corneal staining were extracted.
Majority of the datasets (12 datasets) showed superiority of HY over the control arm, with most (7 datasets) demonstrating statistical significance. Only 5 datasets demonstrated superiority of the control eyedrop and none had statistical significance. The superiority of HY was reported when compared against all 5 different controls – vehicle, PVA, NS, cellulose and carbomer-based eyedrops.
Ocular staining has been used to monitor the severity and progression of DES. Sodium fluorescein permeates intercellular space associated with epithelial cellular disruption; rose bengal stains devitalized cells and mucus through breaks in the tear film; lissamine green stains dead and degenerate cells. The action of HY on CD44 as well as its anti-inflammatory effects may therefore assume greater significance here, having been shown to improve corneal epithelial repair, cellular migration and stabilization of the ocular barrier.
Unfortunately, analyses of this outcome measure is limited by the lack of standardized methodologies and stains. In addition, while some used named grading systems such as the Oxford Scheme and Van Bijsterveld Scoring System, the majority did not specify any grading system. Given this heterogeneity, we attempted to limit our data extraction to results reflecting only cornea staining (with or without the conjunctiva) with fluorescein.
Non-Invasive Tear Breakup Time (NITBUT)
2 studies8, 13 examined NIBUT, comparing 0.1–0.15% HY to CMC and carbomer. The 2 studies utilized the Hir-Cal Grid with a keratometer8 or the Tearscope Plus (Keeler Limited, Windsor, UK). Only Johnson et al.13 reported their results and did not find a statistically nor clinically significant difference between the effects of both arms on NITBUT.
Tear Osmolarity
5 studies7, 8, 18, 22 examined tear osmolarity, with a total of 6 head-to-head comparison datasets. Majority of the control eyedrops were cellulose-based. HY was shown to be superior in 3 of the 5 datasets, with 1 dataset demonstrating statistical significance.
Studies exhibited a range of results. Benelli et al.10 demonstrated improvement in all arms within the first 30 days, while showing superiority of HY. Results across day 15 to day 60 from Iester et al.18 similarly favoured HY. The remaining studies did not show any difference in effect between groups across 30 days to 3 months.
Studies used different measurement techniques and varying concentrations of eyedrops. Studies which demonstrated superiority of HY appeared to use higher concentrations (0.2 to 0.4%) compared to the other studies (0.1 to 0.18%). The varying hypotonicity of eyedrops may also influence the tear osmolarity results25.
Tear osmolarity is a worthwhile outcome measure in any DES study, having been suggested to be the most objective test for DES26. While an unclear cut-off value for the diagnosis of DES has previously limited the usefulness of this test26, the diagnostic threshold of ≥316 mOsm/L has now been validated27. While previous assessment methods were laboratory-based and time-consuming, recent ones are easy-to-use and require minimal tear sample, as that utilized by Benelli et al.10. Finally, from the pathophysiological perspective, tear osmolarity indirectly assesses the rate of tear secretion and loss by evaporation28 resulting from an unstable tear film or reduced volume, therefore representing a meaningful indicator of DES.
Tear Meniscus
3 studies17, 21, 23 examined tear meniscus as an outcome measure, using 0.1% HY as the study eyedrop and 1–1.4% PVA as controls. Laflamme et al.21 used an arbitrary scale based on assessment of the meniscus as ‘normal’ or ‘absent’ and showed superiority of 1.4% PVA, but with only a small sample size not achieving statistical significance. MacDonald et al.16 reported that the tear menisci of subjects in both arms remained unchanged throughout the study.
Conjunctival Impression
5 studies11, 14, 15, 18, 22 examined conjunctival impression – with 2 studies11, 14 utilising flow cytometry and the other 3 studies15, 18, 22 grading the appearance of epithelial and goblet cells.
Sanchez et al.11 and Brignole et al.14 utilised flow cytometry and demonstrated underexpression of HLA-DR in both the HY and control arms, with Sanchez et al. demonstrating greater underexpression in the carmellose arm. This study also showed, without statistical significance, a trend toward decreased expression of other inflammatory markers, including CD11b and CD3. Brignole et al.14 took its first measurements at day 56 and demonstrated a greater decrease in CD44 in the HY group compared to the carboxymethylcellulose (CMC) group. This study further assessed markers understood to be protective in nature – MUC5AC, CD63 and UIC2 – and found these to be increased in both groups, although without statistical significance.
Aragona et al.15, Iester et al.18 and Nelson et al.22 utilized impression cytology scores to reflect qualitative analysis of epithelial and goblet cells. Aragona et al. and Nelson et al. graded specimens utilizing a common method29, while Iester et al. used a different 3-point average scoring system.
Aragona et al.15 demonstrated statistically significant results only after 3 months of treatment, with saline-treated eyes having higher cytology scores (less keratinised basophilic cytoplasm, greater nucleus/cytoplasm ratio and increased goblet cells) compared to the HY arm. This was postulated to be due to the detrimental long-term effect of saline on the ocular surface. Iester et al.18 showed a statistically significant improvement in impression cytology in the HY arm compared to the hydroxypropylmethylcellulose arm at day 90. Nelson et al.22 could not demonstrate any inter-group differences, however showed a statistically significant improvement from baseline in the HY group at 8 weeks.
The late effects of the eyedrops on conjunctival impression cytology and flow cytometry are unsurprising given the long-term nature of cellular turnover and molecular production. This lies beyond the scope of this study, which extracted data up to only 5 weeks after initiation of eyedrops.
Corneal Topography
2 studies10, 14 utilized corneal topography – Benelli et al.10 measured the effect of different eyedrops on wavefront aberrometry while Brignole et al.14 measured corneal surface regularity index (SRI). Brignole et al. demonstrated better SRI outcomes for the HY group, suggesting the presence of greater uniformity in ocular surface protection by HY compared to CMC. Benelli et al.10 showed better outcomes for CMC in wavefront aberrometry, however results were not significant.
Visual Acuity
4 studies6, 10, 22, 23 examined visual acuity (VA) as an outcome measure, with a total of 6 head-to-head comparison datasets. Majority of the studies reported neither their results nor methodologies in detail. Only Benelli et al.10 described VA testing under controlled, standardized environments and showed that HY was superior over CMC and HP Guar, However, this was not statistically significant.
VA is a useful patient-centered outcome and improvement may result from a better tear film quality and ocular surface. However, VA is unavoidably affected by numerous other ocular factors, presenting an inherent limitation in its usefulness as an efficacy measure for ocular surface treatment.
Discussion
The studies reviewed in this paper demonstrated an improvement from baseline across most of the outcome measures for both HY and non-HY based lubricants. However, neither qualitative nor quantitative analyses could reveal consistent nor clinically significant superiority of one treatment arm over the other.
Meta-analyses for SH I and TBUT data revealed that while HY-based preparations appeared superior in improving SH I, non-HY based preparations were more effective in improving TBUT.
However, it should be noted that the SMDs between preparations were small - less than 1mm for SH I (0.238mm) and less than 1 second for TBUT (0.566 seconds). These differences, while statistically significant, are unlikely to have clinical significance – firstly, poor repeatability has been demonstrated for measurements of both SH I and TBUT under various conditions24, 30 and between measuring techniques24. A difference of 3 seconds may be expected between any two separate measurements of TBUT24, suggesting that any difference of less than 3 seconds may not be clinically significant. Secondly, some have suggested that the variability in the SH I test (without anesthesia) is likely to be greater than the SH II (with anesthesia) test as SH I is influenced by reflex, in addition to baseline tear secretion31. Finally, these small differences are unlikely to have significant effect on patient symptoms, given the numerous inherent and environmental factors which have been shown to influence TBUT and SH I measurements32.
As such, the small magnitude of difference for both TBUT and SH I found in the studies we analysed is likely to be well within the expected variability for both outcome measures.
This may reflect true comparability between HY and non-HY preparations. Individually, both HY and non-HY preparations have been demonstrated in most studies to be effective. Their respective mechanisms of action appear to overlap, and it is unsurprising that both would exhibit efficacy in improving both TBUT and SH I, given the basis of these tests.
The effect of HY in the treatment of DES is likely the result of various mechanisms of action. Firstly, in-vitro studies29 have demonstrated that HY inactivates the CD44 adhesion molecule, a receptor of HY found to be over expressed in cornea and conjunctiva cells of subjects with DES. This HY-CD44 interaction has been shown to stabilise the ocular surface barrier and tear film33, creating a favourable ocular surface microenvironment which increases cell adhesion and motility34 and promotes cellular migration35. Secondly, HY has been postulated to have localised anti-inflammatory effects – particularly in patients with at least moderate dry eyes and superficial keratitis36. Thirdly, the high viscosity of HY reduces friction between the cornea and eyelids during extraocular movements and blinking, therefore reducing mechanical damage of the cornea33. Fourthly, HY has significant water-retentive properties – with an affinity of 1000-fold its own weight. This increases ocular surface wettability37 and reduces tear evaporation38.
It is important to realize that many of the comparison groups in published studies are not entirely passive controls but do share some mechanisms of action with HY, which may conceivably improve SH I and TBUT. Carbomer, methylcellulose and PVA are all viscosity-enhancing agents used in eyedrop preparations. Methylcellulose is viscous, anionic and possesses significant bioadhesive characteristics39, 40 which increase the tear retention time41. PVA has film-forming, emulsifying, adhesive properties42 and at the same viscosity, produces a thicker film than other polymers43.
The insignificant difference between HY and non-HY preparations may also be related to the inherent lack of precision and specificity of the SH and TBUT tests. This may also explain the apparent efficacy of placebo and vehicle preparations.
SH I (without anesthesia) reflects both reflex and basal aqueous tear production and remains a common test for diagnosis and monitoring of DES. Unfortunately, this test possesses numerous limitations. These include low reproducibility, low sensitivity and specificity, lack of a standardized site of paper strip placement, uneven absorption of tears by the strip, uncertainty regarding the relationship between the quantity of fluid absorbed by strips with its wetted length and lack of a standardized method of evaluation of the wetted length44. Fujihara T et al.45 concluded that SH I lacks sufficient sensitivity to be a meaningful test for patients with DES that is less than severe. SH I results may improve even with inactive ‘placebo’ preparations, due to their direct effect in increasing tear volume, reducing osmotic pressure, reducing ocular surface friction, and in diluting pro-inflammatory tear substances.
Unlike SH I, TBUT primarily assesses tear film instability in evaporative dry eye disease41. However, again the ‘break up’ of the film may occur due to various mechanisms, including the diffusion and absorption of the lipid layer into the mucous-aqueous interface46 or the rupture of the mucous layer at its weakest spots. Like SH I, TBUT has been labelled inaccurate and non-reproducible47, with lack of a standardized procedure for fluorescein application. It may be poorly associated with subjective dry eye symptoms48.
Qualitative analyses suggest the superiority of HY over the respective control arms in the assessment of SH I, ocular staining, symptoms and tear osmolarity. Neither appeared to be superior in conjunctival impression results. This study did not attempt to draw conclusions from the observation of visual acuity, tear meniscus, NITBUT and corneal topography results, as these outcomes were reported in less than 4 studies each.
The main limitation of our study pertains to the heterogeneity of included studies. Studies differed in study design (parallel vs. crossover), concentration of HY and control eyedrops, eyedrop application regime, follow up duration, baseline characteristics of subjects (differences in age, gender and severity of DES), definition of DES itself49, 50, control eyedrop, as well as the presence of preservatives in the preparations. All these factors may influence the results of this review.
This study has attempted to mitigate this heterogeneity, to enhance the meaningfulness and applicability of the results in this review. Firstly, key characteristics for each study are detailed in Table 2 to allow better contextualization of our results. Secondly, all studies used a HY concentration of at least 0.1%, previously demonstrated as the minimum concentration to have an effect on patients with DES51. Thirdly, this review has attempted to ensure that data extracted across all studies is as homogenous as possible. For example, data has been restricted to that obtained at the earliest time point after the first week, but no later than 35 days after eyedrop instillation. In addition, only studies deemed to have utilized a ‘non-active’ compound in their control arm have been included.
The second limitation of our study pertains to the nature of tests for DES. The lack of a standardized protocol for DES tests has resulted in studies differing on methodologies as well as the quantification of results for the same outcome measure. The limitations of SH I and TBUT have been described above. These factors may confound the results of a meta-analysis which assumes homogeneity in the methodology of outcome measures across various studies52.
Thirdly, most of the studies did not compare HY against its pure vehicle. The differences in molecular weight, tonicity, preservatives and mineral composition between HY and non-HY eyedrops may further influence results53, 54.
Finally, in the interest of reducing heterogeneity among selected studies, data from a relatively narrow window post-initiation of eyedrops was included for analysis. However, depending on the outcome measure, the effect of different lubricant preparations may be observed at other time points. For example, owing to its unique tear stabilizing properties, HY may reduce symptoms and alleviate keratitis within a shorter time than other preparations13, 52. Differences in effect on conjunctival impression and cytology are likely to manifest only later. Therefore, it should be emphasized that this review compares only the short-term/early efficacies of the various artificial tear preparations.
Conclusion
This review examined 18 clinical trials comparing the efficacy of HY against alternative lubricant preparations. Superiority of one preparation over another could not be consistently demonstrated across the range of outcome measures. While meta-analyses demonstrate that HY may provide superior benefit in SH I and non-HY preparations may be superior in improving the TBUT, results were not clinically significant. Overall, this may reflect true comparability in efficacy between HY-based and non-HY preparations. Heterogeneity across studies presents the main limitation to this study, suggesting the need for a large RCT with standardized protocols to properly evaluate the comparative efficacy of artificial tear preparations.
Methods
Literature Search Strategy
An electronic literature search was carried out through the PubMed, Cochrane Central Register of Controlled Trials and Scopus databases from inception up to May 2016. Databases were searched using the terms (“hyaluronic acid” OR “hyaluronate”) AND (“dry eye” OR “keratoconjunctivitis sicca”). Limits were applied to select for only clinical trials performed on human subjects.
Inclusion Criteria
Only in-vivo, prospective, interventional trials examining the effect of HY on the ocular surface were considered for inclusion. Further, studies were included if they: (1) employed at least one HY-only arm; (2) employed at least one non-HY arm, which was determined to use a ‘non-active’ compound (see Table 1) –labelled the ‘control’ arm; (3) had a follow-up period of at least one week after initiation of eyedrops; (4) excluded subjects who were on long-term eyedrops (e.g. glaucoma medications) or contact lens wear; (5) did not perform intra-subject comparison (i.e. comparison of the effect of an eyedrop in one eye and a different eyedrop in the other); (6) were published in English; (7) were not conducted on post-refractive surgery patients.
Both parallel and crossover studies were included in this study.
Study Selection
The abstracts of all studies were read by at least 2 of the authors (BA, JS or PW). Where there were no obvious reasons for immediate exclusion, the full article was reviewed by at least 2 of the above authors. The eligibility of each study was determined unanimously, with strict guidance from the predetermined inclusion criteria. Any discordance regarding a study’s suitability for inclusion was resolved through discussion with the senior author (LT).
Data Extraction
Relevant information and data from the studies were extracted and entered into 3 databases.
The first database included the name of the first author, year of publication, country of study and other study characteristics such as randomization, masking, total duration of follow-up, mean/median age of subjects, severity of DES, % preparation of HY and control eyedrop used (Table 2).The outcome measures obtained in each study were summarized (Table 3).
The second database included results of the outcome measures from the studies, including the number of subjects analysed for each outcome measure, the arm which demonstrated greater comparative efficacy as well as the difference in treatment effect and p-value, if provided in the study. In crossover trials with more than one test period of the same eyedrop medication, only the results of the first trial were included for analysis (unless the results were that from analysis which had already included the consequent test periods).
For studies which had multiple data/outcome measurement points, only the earliest measurement taken between 1 to 5 weeks post-commencement of eye drops was used for analysis. Any measurements taken after 5 weeks post-commencement of eye drops were excluded from data extraction and analysis. Studies with multiple control eyedrop arms or separate results for right and left eyes of subjects have their results presented as separate data entries, hereafter referred to as ‘datasets’. Only results of outcome measures which were examined in 2 or more studies are presented (Supplementary Tables S1–11). This is with the exception of the outcome measure of “safety”. Although “safety” was examined in 8 studies, this outcome measure was deemed too heterogenous, with too large a range of specific “safety” parameters for meaningful comparison and analysis.
The final database extracted the quantitative results reported from randomized controlled trials (RCTs) which studied SH I and/or TBUT. Further meta-analysis was conducted for studies which reported both baseline and follow-up values and/or the change from baseline to follow-up values, for both the HY and non-HY arms. Corresponding authors of studies which did not report the complete set of data as above were contacted via their correspondence email addresses, in an attempt to obtain the missing data. For crossover studies19, 21, the authors agreed that only data from the first trial and the right eye (where applicable)19 was included for analysis. For studies with more than one non-HY intervention group6, 7, 3 in order to avoid unit-of-analysis error, non-HY groups were combined into a single group and compared against the HY treatment arm.
Statistical Analysis
Meta-analyses for both SH I and TBUT as outcome measures were performed with MedCalc for Windows, Version 16.8 (MedCalc Software, Ostend, Belgium). Where the standard deviation of change was not reported in the study, it was imputed with guidance from the Cochrane Handbook for Systematic Reviews of Interventions 55, with Corr, the imputed correlation coefficient, taken as 0.5 in this study.
Standardized mean difference (SMD) was used for the continuous data and 95% confidence intervals (CIs) were calculated for summary estimates. A p-value of less than 0.05 was considered statistically significant. The χ2-basedI2 statistic (expressed as a percentage) was used to measure heterogeneity among the trials. An I2 value of more than 50% was considered suggestive of significant statistical heterogeneity. Both fixed and random-effects modelling were applied in analyses.
Risk of Bias Assessment
All the studies included for meta-analysis were assessed for risk of bias using the Cochrane Risk of Bias tool56. This tool evaluates studies for risk of selection bias, performance bias, attrition bias, reporting bias and other types of bias. 2 authors (BA and JS) independently assessed the risk of bias. Discrepancies were resolved by discussion and consultation with the senior author (LT)57.
Funnel plots of the intervention effect estimates were developed and examined for evidence of asymmetry. A symmetric funnel plot reflects less bias while an asymmetric funnel plot may imply possible selection/publication bias, poor reporting of small trials, chance or true heterogeneity.
References
The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop. Ocul Surf, 5(2), 75–92 (2007).
Thoft, R. A. & Friend, J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. 1442–3 (1983).
Baudoin, C. The Pathology of Dry Eye. Surv Ophthalmo. 45, S211–S220 (2001).
Report of the Epidemiology Subcommittee of the International DEWS: The epidemiology of dry eye disease (2007).
Kong, X. et al. Sodium hyaluronate’s effect on xerophthalmia: a meta-analysis of randomized controlled trials. Curr Med Res Opin. 32(3), 477–84 (2016).
Baeyens, V., Bron, A. & Baudouin, C. Vismed/Hylovis Study Group. Efficacy of 0.18% hypotonic sodium hyaluronate ophthalmic solution in the treatment of signs and symptoms of dry eye disease. J Fr Ophtalmol. 35(6), 412–9 (2012).
Baudouin, C. et al. Randomized, phase III study comparing osmoprotective carboxymethylcellulose with sodium hyaluronate in dry eye disease. Eur J Ophthalmol. 22(5), 751–61 (2012).
McCann, L. C., Tomlinson, A., Pearce, E. I. & Papa, V. Effectiveness of artificial tears in the management of evaporative dry eye. Cornea. 31(1), 1–5 (2012).
Lee, J. H., Ahn, H. S., Kim, E. K. & Kim, T. Efficacy of Sodium Hyaluronate and Carboxymethylcellulose in Treating Mild to Moderate Dry Eye Disease. Cornea. 30(2), 175–179 (2011).
Benelli, U., Nardi, M., Posarelli, C. & Albert, T. G. Tear osmolarity measurement using the TearLab Osmolarity System in the assessment of dry eye treatment effectiveness. Cont Lens Anterior Eye. 33(2), 61–7 (2010).
Sanchez, M. A. et al. Comparative analysis of carmellose 0.5% versus hyaluronate 0.15% in dry eye: a flow cytometric study. Cornea. 29(2), 167–71 (2010).
Vogel, R. et al. Sodium Hyaluronate Ophthalmic Solution Study Group. Demonstration of efficacy in the treatment of dry eye disease with 0.18% sodium hyaluronate ophthalmic solution (vismed, rejena). Am J Ophthalmol. 149(4), 594–601 (2010).
Johnson, M. E., Murphy, P. J. & Boulton, M. Effectiveness of sodium hyaluronate eyedrops in the treatment of dry eye. Graefes Arch Clin Exp Ophthalmol. 244(1), 109–12 (2006).
Brignole, F., Pisella, P. J., Dupas, B., Baeyens, V. & Baudouin, C. Efficacy and safety of 0.18% sodium hyaluronate in patients with moderate dry eye syndrome and superficial keratitis. Graefes Arch Clin Exp Ophthalmol. 243(6), 531–8 (2005).
Aragona, P., Papa, V., Micali, A., Santocono, M. & Milazzo, G. Long term treatment with sodium hyaluronate-containing artificial tears reduces ocular surface damage in patients with dry eye. Br J Ophthalmol. 86(2), 181–4 (2002).
Benitez, C., Concha, A. & Garcia-Sanchez, J. Corneal epithelial permeability and dry eye treatment. Adv Exp Med Biol. 506(Pt A), 703–6 (2002).
McDonald, C. C., Kaye, S. B., Figueiredo, F. C., Macintosh, G. & Lockett, C. A randomised, crossover, multicentre study to compare the performance of 0.1% (w/v) sodium hyaluronate with 1.4% (w/v) polyvinyl alcohol in the alleviation of symptoms associated with dry eye syndrome. Eye (Lond). 16(5), 601–7 (2002).
Iester, M. et al. Improvement of the ocular surface using hypotonic 0.4% hyaluronic acid drops in keratoconjunctivitis sicca. Eye (Lond). 14(Pt 6), 892–8 (2000).
Condon, P. I. et al. Double blind, randomised, placebo controlled, crossover, multicentre study to determine the efficacy of a 0.1% (w/v) sodium hyaluronate solution (Fermavisc) in the treatment of dry eye syndrome. Br J Ophthalmol. 83(10), 1121–4 (1999).
Sand, B. B., Marner, K. & Norn, M. S. Sodium hyaluronate in the treatment of keratoconjunctivitis sicca. A double masked clinical trial. Acta Ophthalmol (Copenh). 67(2), 181–3 (1989).
Laflamme, M. Y. & Swieca, R. A comparative study of two preservative-free tear substitutes in the management of severe dry eye. Can J Ophthalmol. 23(4), 174–6 (1988).
Nelson, J. D. & Farris, R. L. Sodium hyaluronate and polyvinyl alcohol artificial tear preparations. A comparison in patients with keratoconjunctivitis sicca. Arch Ophthalmol. 106(4), 484–7 (1988).
Limberg, M., McCaa, C., Kissling, E. & Kaufman, H. Topical Application of hyaluronic acid and chondroitin sulfate in the treatment of dry eyes. American Journal of Ophthalmology. 103-2, 194–197 (1987).
Elliott, M., Fandrich, H., Simpson, T. & Fonn, D. Analysis of the repeatability of tear break-up time measurement techniques on asymptomatic subjects before, during and after contact lens wear. Cont Lens Anterior Eye. 21(4), 98–103 (1998).
Benelli, W. R. M., Folberg, R., Krachmer, J. H. & Holland, E. J. The treatment of aqueous deficient dry eye with removable punctal plugs: a clinical and impression-cytologic study. Ophthalmology. 84, 514–8 (1987).
Gilbard, J. P., Farris, R. L. & Santamaria, J. II. Osmolarity of tear microvolumes in keratoconjunctivitis sicca. Arch Ophthalmol. 96, 677–681 (1978).
Tomlinson, A., Khanal, S., Ramaesh, K., Diaper, C. & McFadyen, A. Tear film osmolarity: determination of a referent for dry eye diagnosis. Invest Ophthalmol Vis Sci. 47, 4309–15 (2006).
Gilbard, J. P. & Farris, R. L. Tear osmolarity and ocular surface disease in keratoconjunctivitis sicca. Arch Ophthalmol. 97, 1642–1646 (1979).
Nelson, J. D., Havener, V. R. & Cameron, J. D. Cellulose acetate impressions of the ocular surface. Arch Ophthalmol. 101, 1868–72 (1983).
Kallarackal, G. U. et al. A comparative study to assess the clinical use of Fluorescein Meniscus Time (FMT) with Tear Break up Time (TBUT) and Schirmer’s tests (ST) in the diagnosis of dry eyes. Eye (Lond). 6(5), 594–600 (2002).
Masmali, A., Alqahtani, T. A., Alharbi, A. & El-Hiti, G. A. Comparative study of repeatability of phenol red thread test versus Schirmer test in normal adults in Saudi Arabia. Eye Contact Lens. 40(3), 127–31 (2014).
Hua, R., Yao, K., Hu, Y. & Chen, L. Discrepancy between subjectively reported symptoms and objectively measured clinical findings in dry eye: a population based analysis. BMJ Open. 28, 4 (2014).
Baudouin, F., Brignole, F. & Dupas, B. et al. Reduction in keratitis and CD44 expression in dry eye patients treated with a unique 0.18% sodium hyaluronate solution. (ARVO abstract). Invest Ophthalmol Vis Sci. 42, S32 (2001).
Nishida, T., Nakamura, M. & Mishima, H. et al. Hyaluronan stimulates corneal epithelial migration. Exp Eye Res. 53, 753–8 (1991).
Inoue, M. & Katakami, C. The effect of hyaluronic acid on corneal epithelial cell proliferation. Invest Ophthalmol Vis Sci (1993).
Stern, M. E., Beuerman, R. W. & Fox, R. I. et al. The pathology of dry eye: the interaction between the ocular surface and lacrimal gland. Cornea. 17, 584–9 (1998).
Nakamura, M., Hikida, M. & Nakano, T. et al. Characterization of water retentive properties of hyaluronan. Cornea. 12, 433–6 (1993).
Balazs EA: Amino sugar-containing macro- molecules in the tissues of the eye and ear, In: Amino Sugar: Chemistry and Biology of Compound Containing Amino Sugar, Vol 2 A. Distribution and Biological Role. Academic Press Inc. 2, 401–460 (1965).
Peppas, N. A. & Buri, P. A. Surface, interfacial and molecular aspects of polymer bioadhesion on soft tissues. J Controll Release. 2, 257–275 (1985).
Hunt, G., Kearney, P. & Kellaway, I. W. Mucoadhesive Polymers in Drug Delivery Systems Fundamentals and Techniques. Wein heim, Germany: VCH. 1, 180–199 (1987).
Methodologies to diagnose and monitor dry eye disease: Report of the Diagnostic Methodology Subcommittee of the International Dry Eye Work Shop (2007). Ocul Surf. 5(2), 108–52 (2007).
Fromageau, J. et al. Characterization of PVA cryogel for intravascular ultrasound elasticity imaging. IEEE Transactions on Ultrasonics, Ferroelectrics and Frequency Control. 50(10), 1318–1324 (2003).
Benedetto, D., Shah, D. & Kaufmann, H. The instilled fluid dynamics and surface chemistry of polymers in the preocular film. Invest Ophthalmol. 14, 887–902 (1975).
Cho, P. & Yap, M. Schirmer test. I. A review. Optom Vis Sci. 70, 152–6 (1993).
Tsubota, K., Xu, K. P. & Fujihara, T. et al. Decreased reflex tearing is associated with lymphocitic infiltration in lacrimal glands. J Rheumatol. 23, 313–20 (1996).
Holly, F. J. & Lemp, M. A. Wettability and wetting of corneal epithelium. Exp Eye Res. 11, 239–250 (1971).
Vanley, G. T., Leopold, I. H. & Gregg, T. H. Interpretation of tear film breakup. Arch Ophthalmol. 95, 445–8 (1977).
Nichols, J. J., Nichols, K. K. & Puent, B. et al. Evaluation of tear film interference patterns and measures of tear break-up time. Optom Vis Sci. 79, 363–9 (2002).
Savini, G. et al. The challenge of dry eye diagnosis. Clinical ophthalmology (Auckland, NZ). 2(1), 31–55 (2008).
Pflugfelder, S. C., Tseng, S. C. & Sanabria, O. et al. Evaluation of subjective assessments and objective diagnostic tests for diagnosing tear-film disorders known to cause ocular irritation. Cornea. 17, 38–56 (1998).
Hamano, T., Horimoto, K., Lee, M. & Komemushi, S. Sodium hyaluronate eyedrops enhance tear film stability. Jpn J Ophthalmol. 40(1), 62–5 (1996).
Foulks, G. N. Challenges and pitfalls in clinical trials of treatments for dry eye. Ocul Surf. 1, 20–30 (2003).
Benitez-del-Castillo, J. M., Aranguez, C. & Garcia-Sanchez, J. Corneal epithelial permeability and dry eye treatment. Adv Exp Med Biol. 506, 703–6 (2006).
Pflugfelder, S. C., Corrales, R. M. & de Paiva, C. S. T helper cytokines in dry eye disease. Exp Eye Res. 117, 118–125 (2013).
Higgins, J. P. T., Green, S. Cochrane Handbook for Systematic Reviews of Interventions. http://handbook.cochrane.org/chapter_16/16_1_3_2_imputing_standard_deviations_for_changes_from_baseline.htm), (Date of access: 16/10/2016) (2011).
Higgins, J. P. T., Green, S. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Collaboration. http://handbook.cochrane.org, (Date of access: 16/10/2016) (2011).
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). http://www.prisma-statement.org/, (Date of access: 16/10/2016) (2015).
Author information
Authors and Affiliations
Contributions
L.T. conceptualized the study and provided supervision throughout the study. B.A., J.S. and P.W. contributed to the literature search and data collection. B.A., J.S. and H.H. contributed to the data and statistical analysis. B.A. and J.S. wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ang, B.C.H., Sng, J.J., Wang, P.X.H. et al. Sodium Hyaluronate in the Treatment of Dry Eye Syndrome: A Systematic Review and Meta-Analysis. Sci Rep 7, 9013 (2017). https://doi.org/10.1038/s41598-017-08534-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-08534-5
This article is cited by
-
Recent advances in ocular lubrication
Friction (2024)
-
A protocol for a single center, randomized, controlled trial comparing the clinical efficacy of 3% diquafosol and 0.1% hyaluronic acid in diabetic patients with dry eye disease
Trials (2023)
-
The Combined Impact of Intense Pulsed Light Combined and 3% Diquafosol Ophthalmic Solution on Evaporative Dry Eye: A Randomized Control Study
Ophthalmology and Therapy (2023)
-
A randomized multicenter evaluation of the efficacy of 0.15% hyaluronic acid versus 0.05% cyclosporine A in dry eye syndrome
Scientific Reports (2022)
-
Dry eye syndrome: comprehensive etiologies and recent clinical trials
International Ophthalmology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.